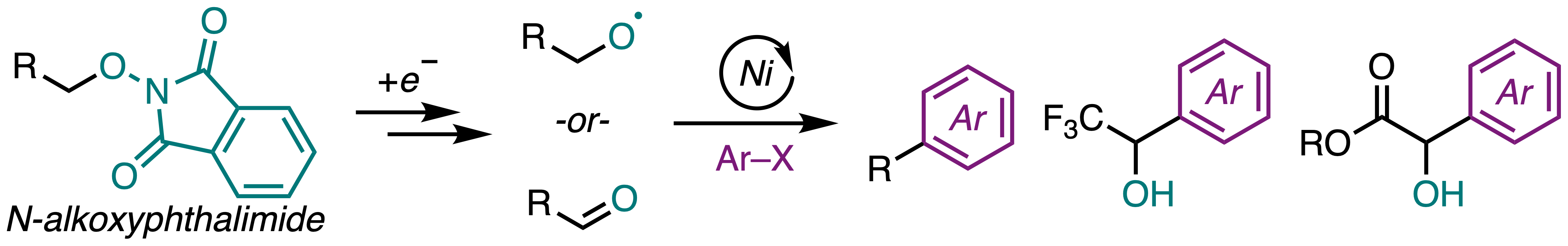50. Pearson, J. W.; Dudra, S. L; Palermo, A. F.; Chiu, B. S. Y; Dang, J.; Gabbey, A. L.; Henson, B. A. B.; Hou, T. R.; Nabavi, N.; and Rousseaux, S. A. L. "Overridding Norish Type II to Access Cyclopropanols." J. Am. Chem. Soc. 2025. DOI: 10.1021/jacs.5c13001
49. Monteith, J. J.; Rousseaux, S. A. L. "Ni-Catalyzed Cross-Electrophile Coupling of N-Alkoxyphthalimides and Aryl Halides." Eur. J. Org. Chem. 2025. DOI: 10.1002/ejoc.202500122.
48. Graham, J. M.; Rousseaux, S. A. L. "Ni-Catalyzed Reductive Cyanation of Alkenyl Tosylates and Triflates." Chem. Commun. 2025, 61, 893–896.
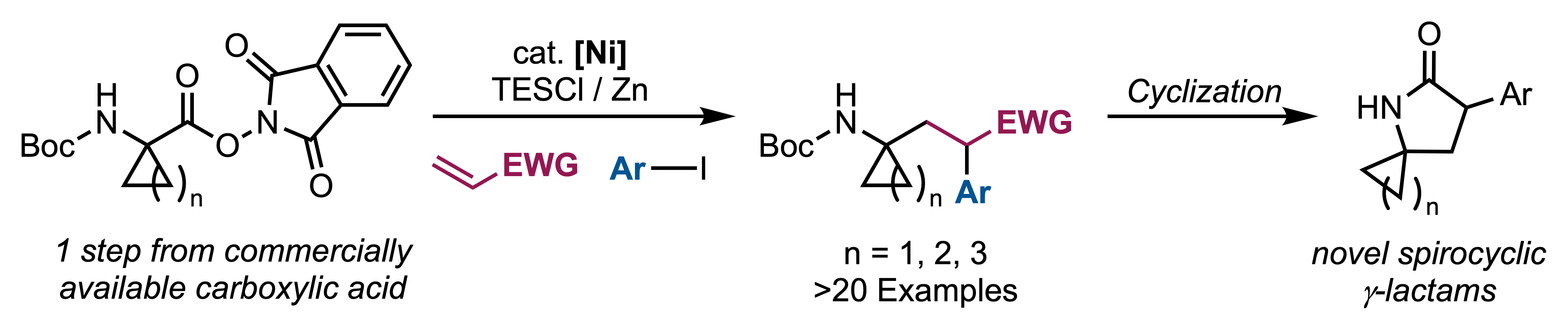
47. Pearson, J. W.; Hou, T. R.; Golijanin, J.; Stewart, P. I.; Choi, E. S.; Gabbey, A. L.; West, M. S.; Rousseaux, S. A. L. "Ni-Catalyzed Reductive 1,2-Alkylarylation of Alkenes for the Synthesis of Spirocyclic gamma-Lactams." Org. Lett. 2024, 26, 5560–5565.
46. Monteith, J. J.; Rousseaux, S. A. L. "A Dual Ni/Photoredox Cross-Coupling Approach toward Mandelic Acids." Org. Lett. 2024, 26, 4566–4570.
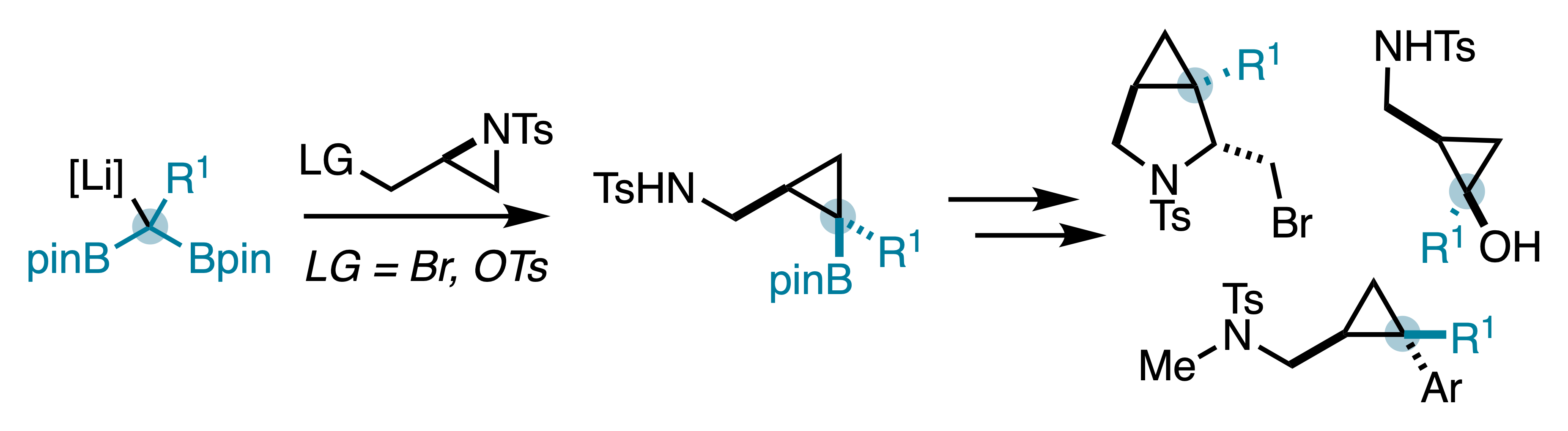
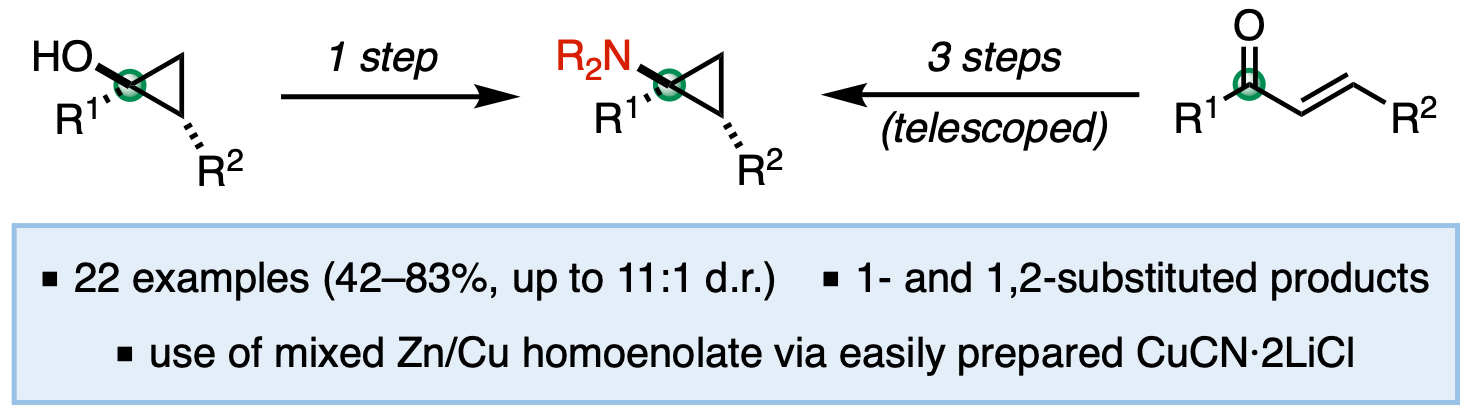
45. McDonald, T. R.; Turner, J. A.; Gabbey, A. L.; Balasubramanian, P.; Rousseaux, S. A. L. "Synthesis of Borylated (Aminomethyl)cyclopropanes Using C1-Bisnucleophiles." Org. Lett. 2024, 26, 3822–3827.
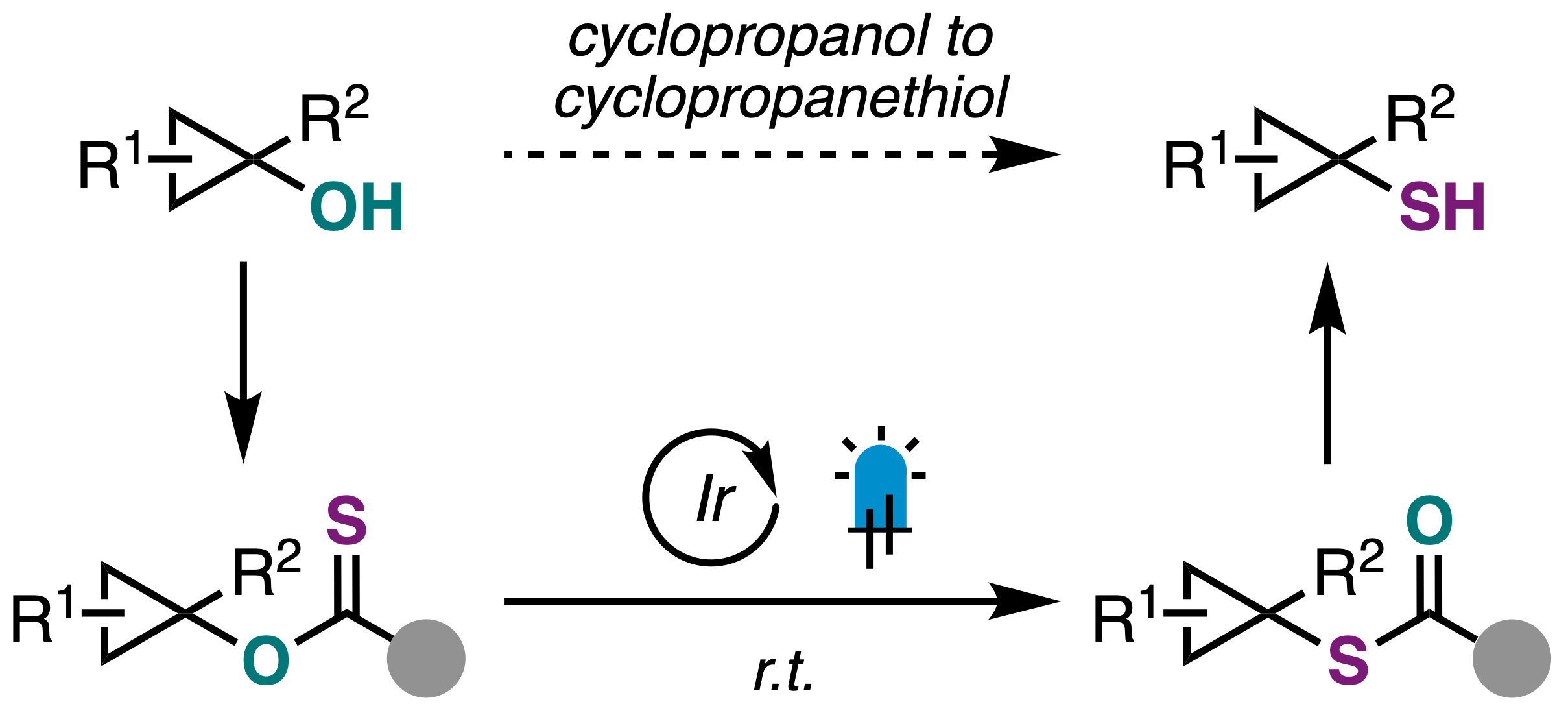
44. Monteith, J. J.; Pearson, J. W.; Rousseaux, S. A. L. "Photocatalytic O- to S-Rearrangement of Tertiary Cyclopropanols." Angew. Chem. Int. Ed. 2024, 63, e202402912.
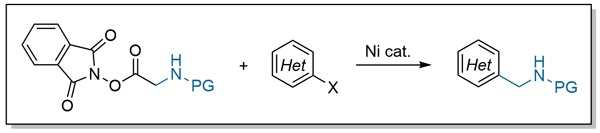
43. Choi, E. S.; Rousseaux, S. A. L.; Huestis, M. P. "Nickel-Catalyzed Synthesis of Benzylamines from (Hetero)aryl Halides and Glycine-Derived N-Hydroxyphthalimide Esters." Synlett 2024, 35, A–E.
42. Michel, N. W. M.; Gabbey, A. L.; Edjoc, R. K.; Fagbola, E.; Hughes, J. M. E.; Campeau, L.-C.; Rousseaux, S. A. L. "Nickel-Catalyzed Reductive Arylation of Redox Active Esters for the Synthesis of α-Aryl Nitriles – Investigation of a Chlorosilane Additive." J. Org. Chem. 2024, 89, 16161–16169. ChemRxiv Preprint: doi.org/10.26434/chemrxiv.14450007.v2.
41. Monteith, J. J.; Rousseaux, S. A. L. "Redox-Active Thiocarbonyl Auxiliaries in Ni-Catalyzed Cross-Couplings of Aliphatic Alcohols." Acc. Chem. Res. 2023, 56, 3581–3594.
40. Palermo, A. F.; Imbriaco, B.; Chan, S. C.; Doan, B. A.; Rousseax, S. A. L. "Asymmetric Cu(I)-Catalyzed Conjugate Borylation of α,β-Unsaturated Acyl Silanes." Synlett, 2023, 34, A–D. DOI: 10.1055/s-0043-1763621.
39. Palermo, A. F.; Chiu, B. S. Y.; Patel, P.; Rousseaux, S. A. L. "Nickel-Catalyzed Reductive Alkyne Hydrocyanation Enabled by Malononitrile and a Formaldehyde Additive" J. Am. Chem. Soc. 2023, 145, 24981–24989.
38. Monteith, J. J.; Rousseaux, S. A. L. "Asymmetric Synthesis of Cyclopropane and Cyclobutane-Containing Small Molecule Pharmaceuticals" Reference Module in Chemistry, Molecular Sciences and Chemical Engineering 2022. DOI: 10.1016/B987-0-32-390644-9.00089-5.
37. Gabbey, A. L.; Scotchburn, K.; Rousseaux, S. A. L. "Metal-Catalysed C–C Bond Formation at Cyclopropanes" Nat. Rev. Chem. 2023, 7, 548–560.
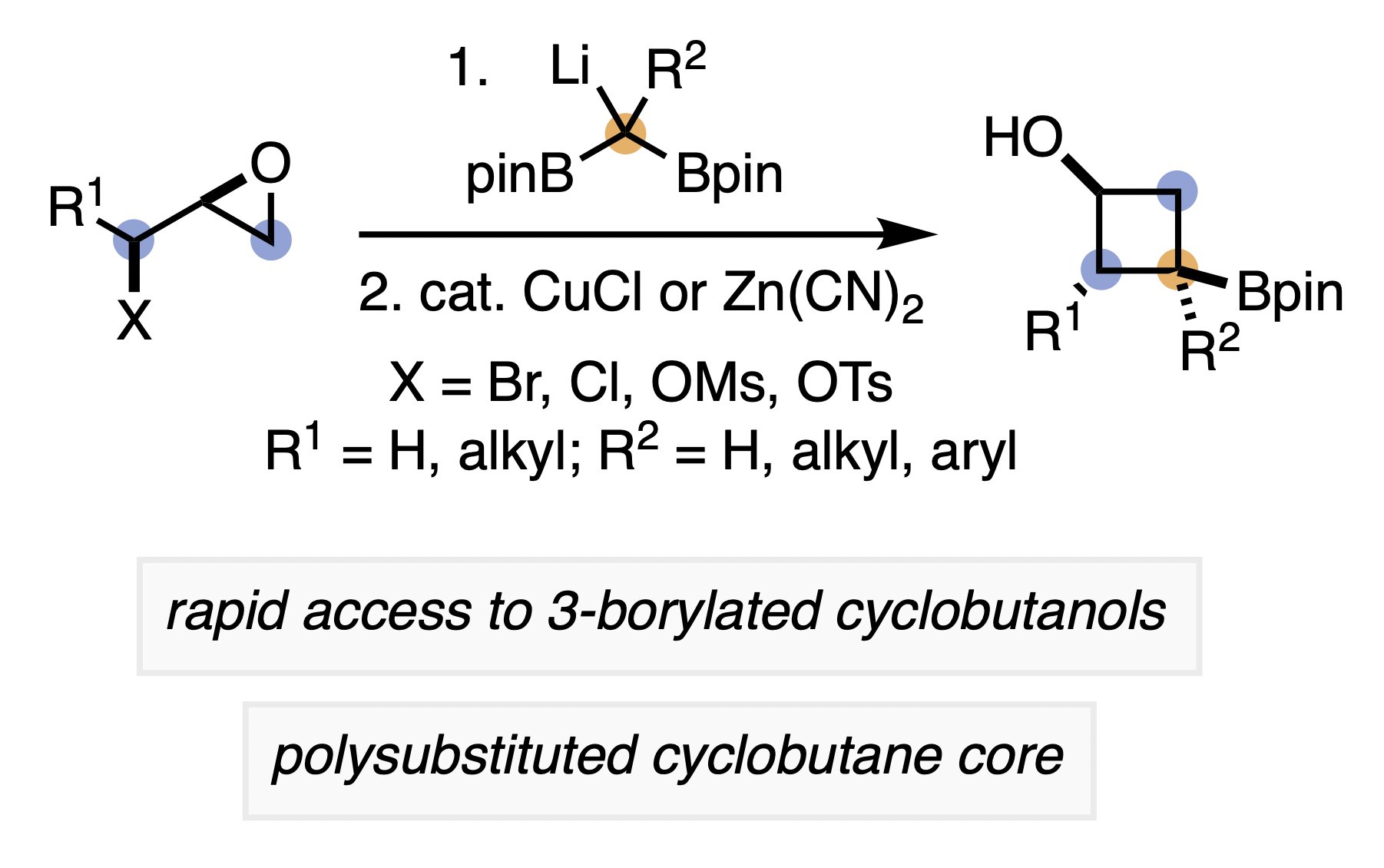
36. McDonald, T. R. M.; Rousseaux, S. A. L. "Synthesis of 3-Borylated Cyclobutanols from Epihalohydrins or Epoxy Alcohol Derivatives" Chem. Sci. 2023, 14, 963–969. ChemRxiv Preprint:10.26434/chemrxiv-2022-99gl2.
35. West, M. S.; Gabbey, A. L.; Huestis, M. P.; Rousseaux, S. A. L. "Ni-Catalyzed Reductive Cross-Coupling of Cyclopropylamines and Other Strained Ring NHP Esters with (Hetero)Aryl Halides" Org. Lett. 2022, 24, 8441–8446. ChemRxiv Preprint: 10.26434/chemrxiv-2022-853zt.

34. West, M. S.; Rousseaux, S. A. L. "Safe, Selective, and Scaleable Carbenes" Science 2022, 377, 580–581.
33. West, M. S.; Pia, J.; Rousseaux, S. A. L. "Synthesis of 1- and 1,2-Substituted Cyclopropylamines from Ketone Homoenolates" Org. Lett. 2022, 24, 5869–5873.

32. Gabbey, A. L.; Michel, N. W. M.; Hughes, J. M. E.; Campeau, L.-C.; Rousseaux, S. A. L. "Synthesis of α-Aryl Secondary Amides via Nickel-Catalyzed Reductive Coupling of Redox-Active Esters" Org. Lett. 2022, 24, 3173–3178.
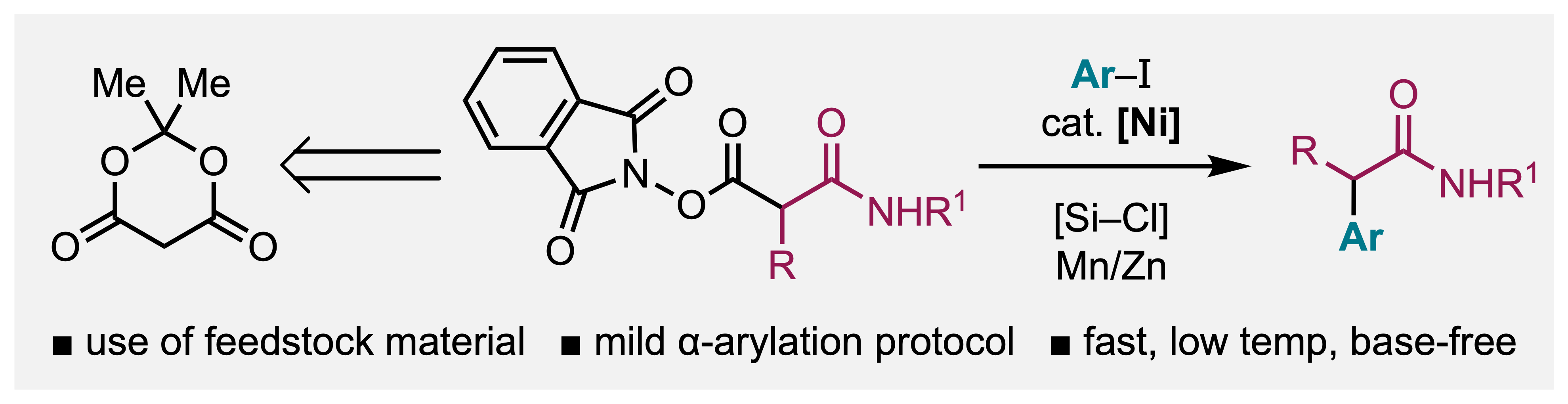
31. Mills, L. R.; Patel, P.; Rousseaux, S. A. L. "Decyanation–(hetero)arylation of malononitriles to access α-(hetero)arylnitriles" Org. Biomol. Chem. 2022, 20, 5933–5937.
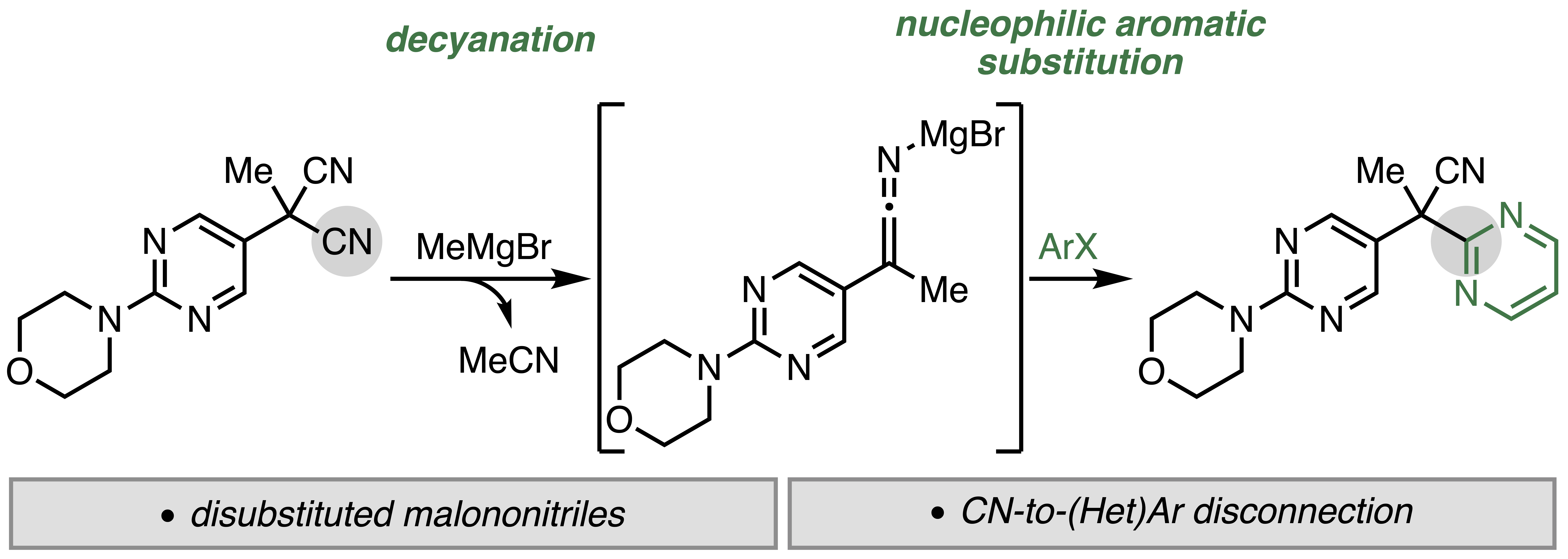
30. Monteith, J. J.; Scotchburn, K.; Mills, L. R.; Rousseaux, S. A. L. "Ni-Catalyzed Synthesis of Thiocarboxylic Acid Derivatives" Org. Lett. 2022, 24, 619–624.
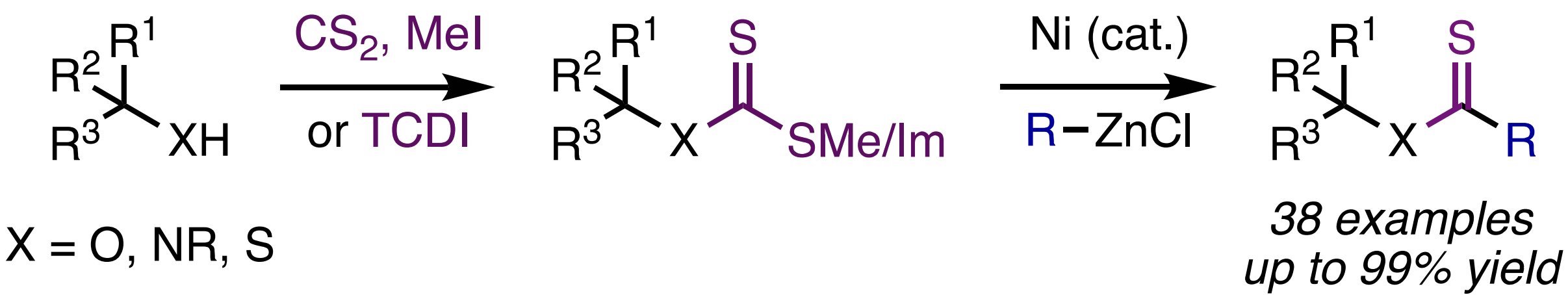
29. Monteith, J. J.; Rousseaux, S. A. L. "Ni-Catalyzed C(sp3)–O Arylation of α-Hydroxy Esters" Org. Lett. 2021, 23, 9485–9489.
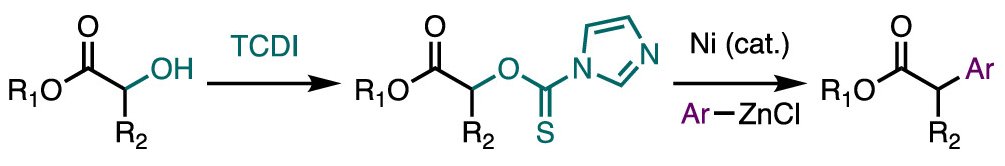
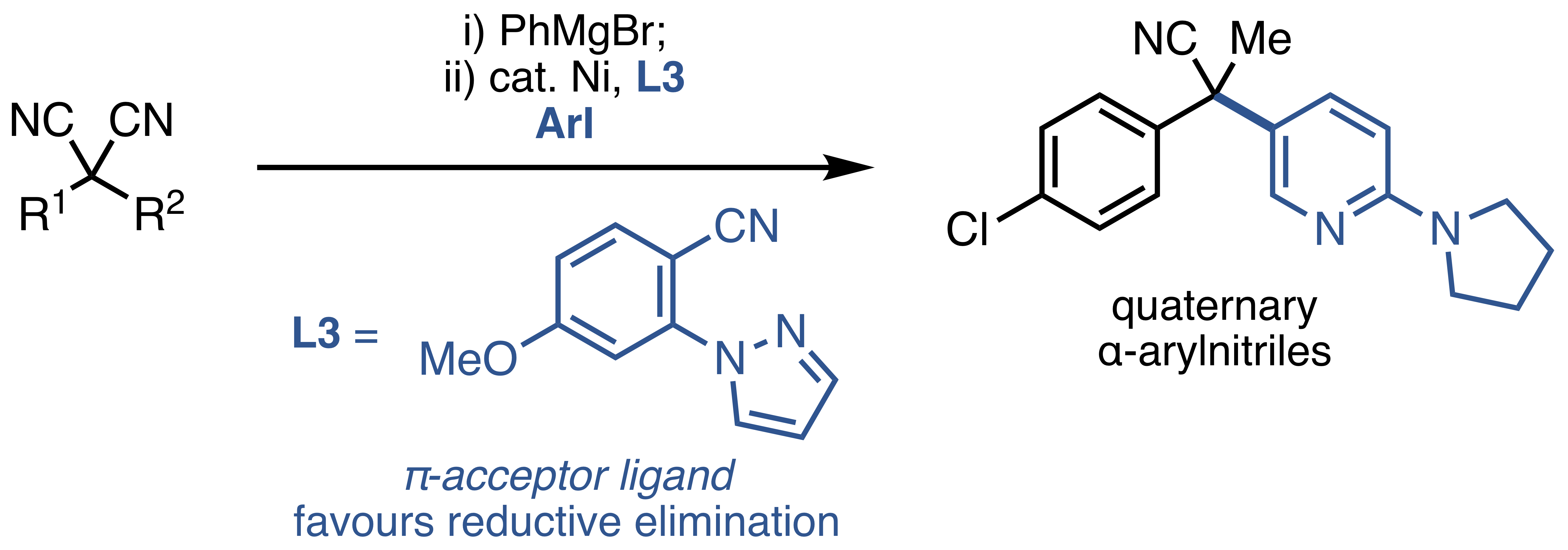
28. Mills, L. R.; Edjoc, R. K.; Rousseaux, S. A. L. "Design of an Electron-Withdrawing Benzonitrile Ligand for Ni-Catalyzed Cross-Coupling Involving Teritary Nucleophiles" J. Am. Chem. Soc. 2021, 143, 10422–10428. ChemRxiv preprint (doi.org/10.26434/chemrxiv.14298665.v1)
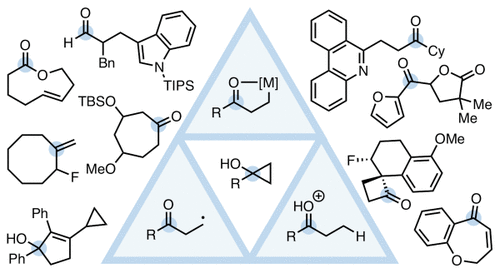
27. McDonald, T. R.; Mills, L. R.; West, M. S.; Rousseaux, S. A. L. "Selective Carbon–Carbon Bond Cleavage of Cyclopropanols" Chem. Rev. 2021, 121, 3–79.
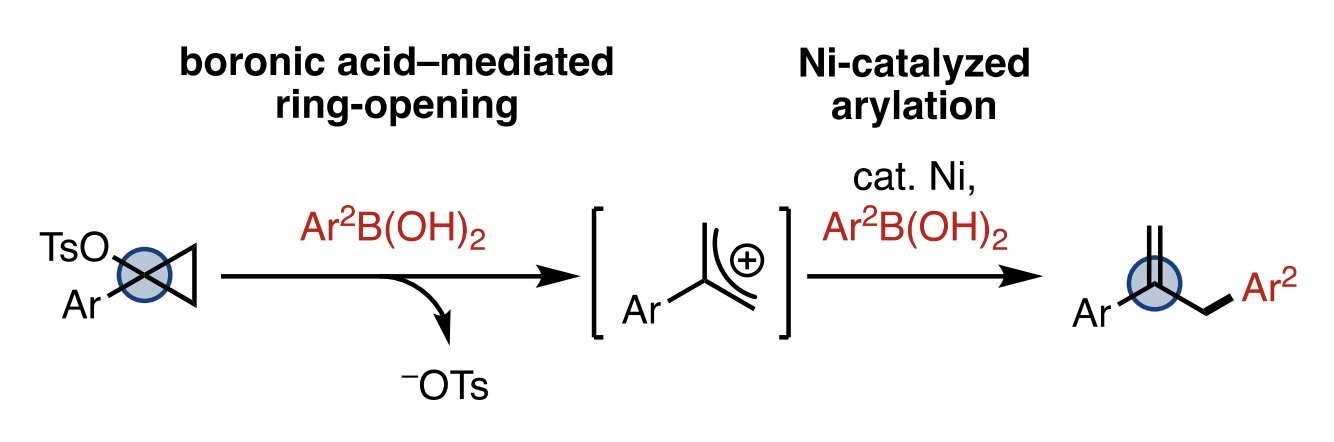
26. Mills, L. R.; Monteith, J. J.; Rousseaux, S. A. L. "Boronic Acid-Mediated Ring-Opening and Ni-Catalyzed Arylation of 1-Arylcyclopropyl Tosylates" Chem. Commun. 2020, 56, 12538–12541.
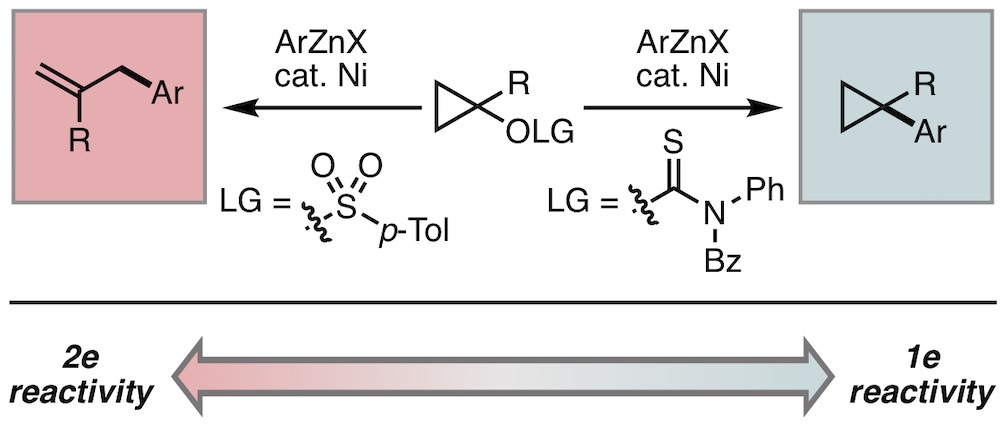
25. Mills, L. R.; Monteith, J. J.; Gomes, G. P.; Aspuru-Guzik, A.; Rousseaux, S. A. L. "The Cyclopropane Ring as a Reporter of Radical Leaving-Group Reactivity in Ni-Catalyzed C(sp3)–O Arylation" J. Am. Chem. Soc. 2020, 142, 13246–13254.
ChemRxiv preprint (doi: 10.26434/chemrxiv.11857905.v1)
24. Mills, L. R.; Graham, J.; Patel, P.; Rousseaux, S. A. L. "Ni-Catalyzed Reductive Cyanation of Aryl Halides and Phenol Derivatives via Transnitrilation" J. Am. Chem. Soc. 2019, 141, 19257–19262.
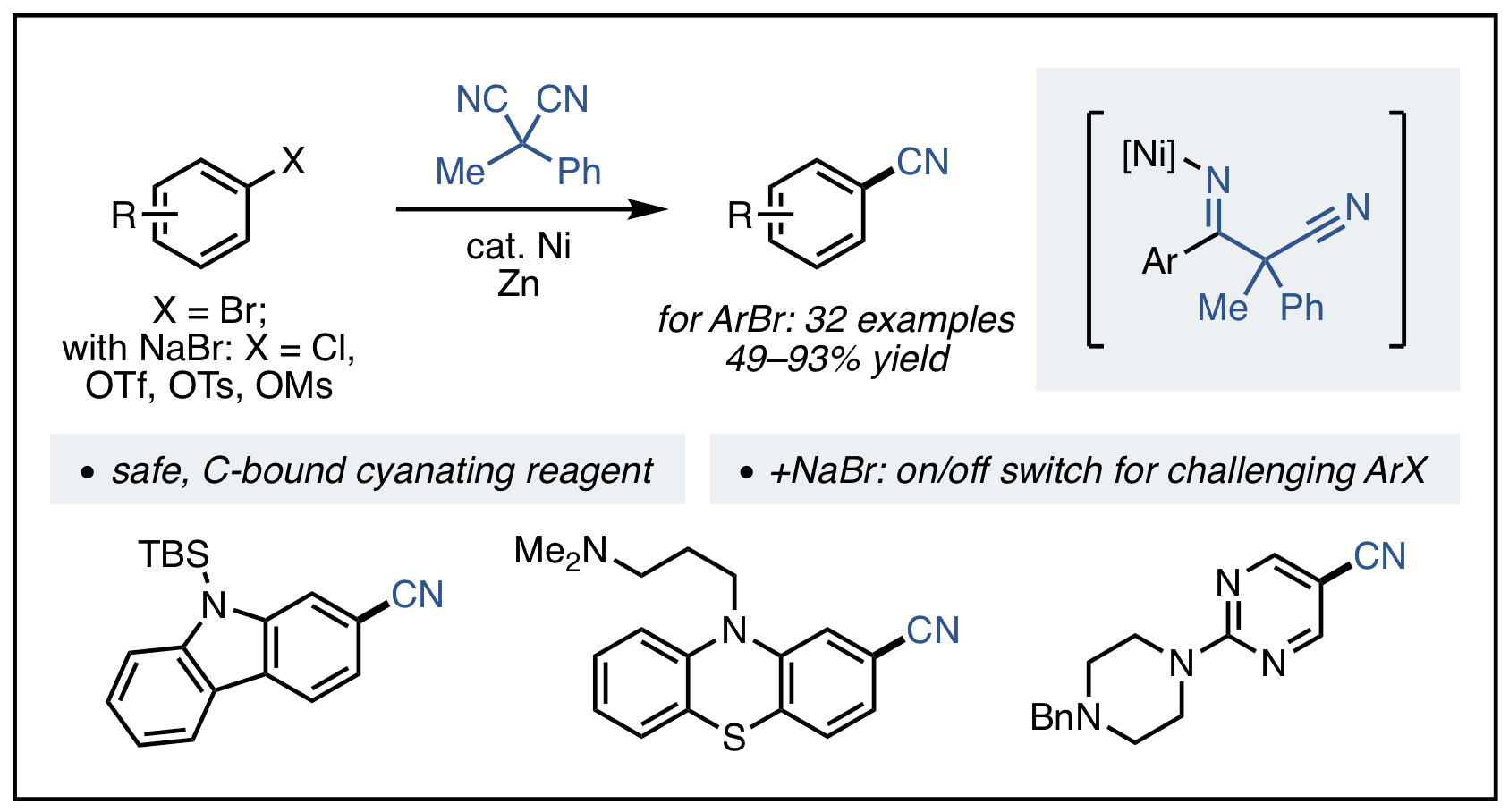
23. Mills, L. R.; Zhou, C.; Fung, E.; Rousseaux, S. A. L. "Ni-Catalyzed β-Alkylation of Cyclopropanol-Derived Homoenolates" Org. Lett. 2019, 21, 8805–8809.

22. West, M. S.; Mills, L. R.; McDonald, T. R.; Lee, J. B.; Ensan, D.; Rousseaux, S. A. L. "Synthesis of trans-2-Substituted-Cyclopropylamines from α-Chloroaldehydes" Org. Lett. 2019, 21, 8409–8413.
ChemRxiv preprint (doi: 10.26434/chemrxiv.9765116.v1)
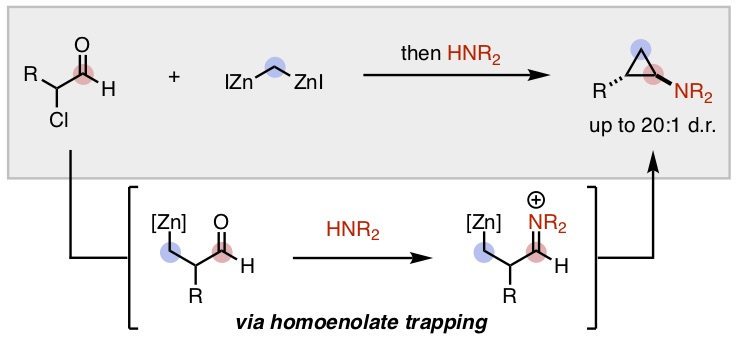
21. Patel, P.; Rousseaux, S. A. L. "Nickel-Catalyzed Amination of α-Aryl Methyl Ethers" Synlett 2020, 31, 492–396. Part of a special issue dedicated to the 2019 Young Investigators Workshop of the Organic Division of EuCheMS.
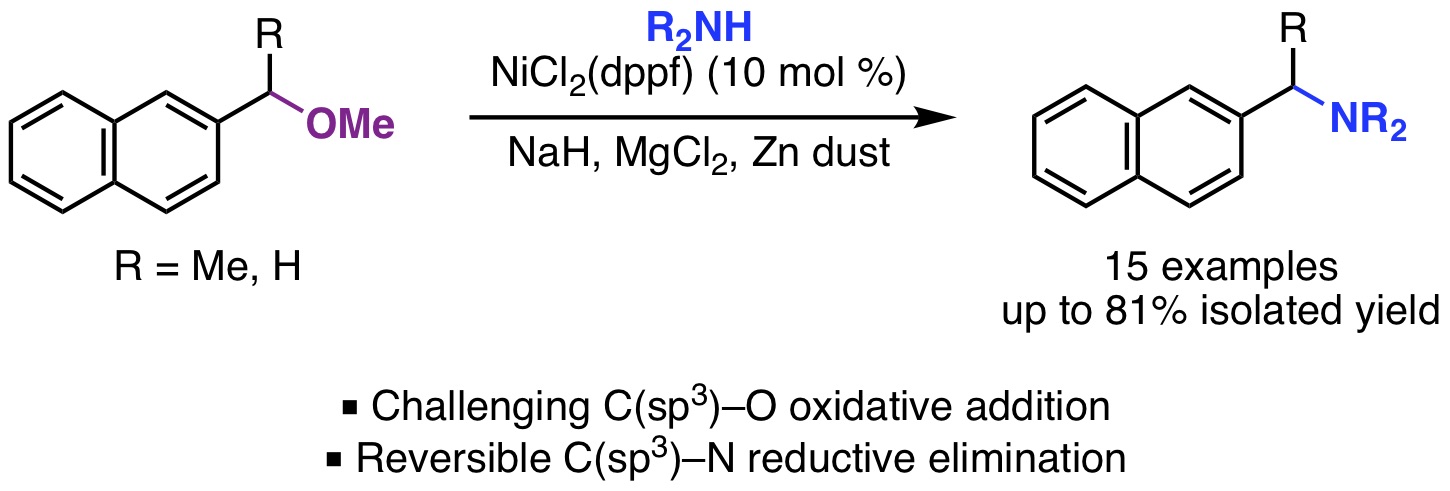
20. Alazet, S.; West, M. S.; Patel, P.; Rousseaux, S. A. L. "Synthesis of Nitrile-Bearing Quaternary Centers by an Equilibrium-Driven Transnitrilation and Anion-Relay Strategy" Angew. Chem. Int. Ed. 2019, 58, 10300-10304.
ChemRxiv preprint (doi: 10.26434/chemrxiv.7834940.v1)
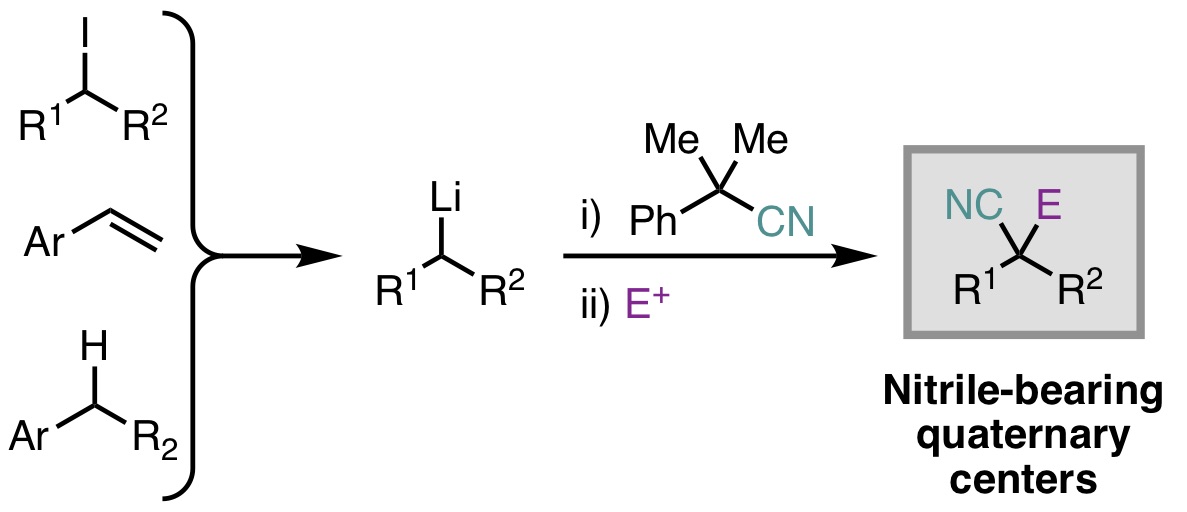
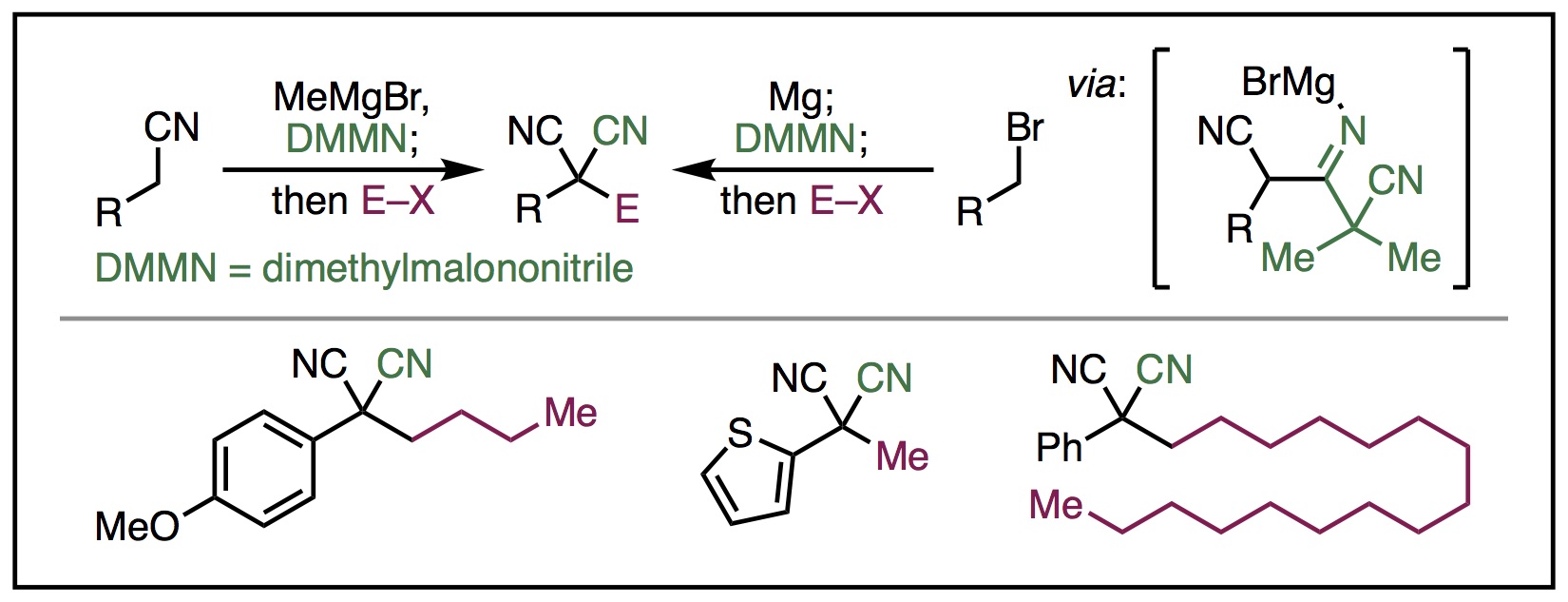
19. Mills, L. R.; Rousseaux, S. A. L. "A one-pot electrophilic cyanation–functionalization strategy for the synthesis of disubstituted malononitriles" Tetrahedron 2019, 75, 4298-4306. Part of a special issue in honor of Prof. Stephen Buchwald's 2018 Tetrahedron Prize for Creativity in Organic Chemistry.
18. Obhi, N. K.; Mallov, I.; Borduas-Dedekind, N.; Rousseaux, S. A. L.; Dicks, A. P. "Comparing Industrial Amination Reactions in a Combined Class and Laboratory Green Chemistry Assignment" J. Chem. Ed. 2019, 96, 93-99.
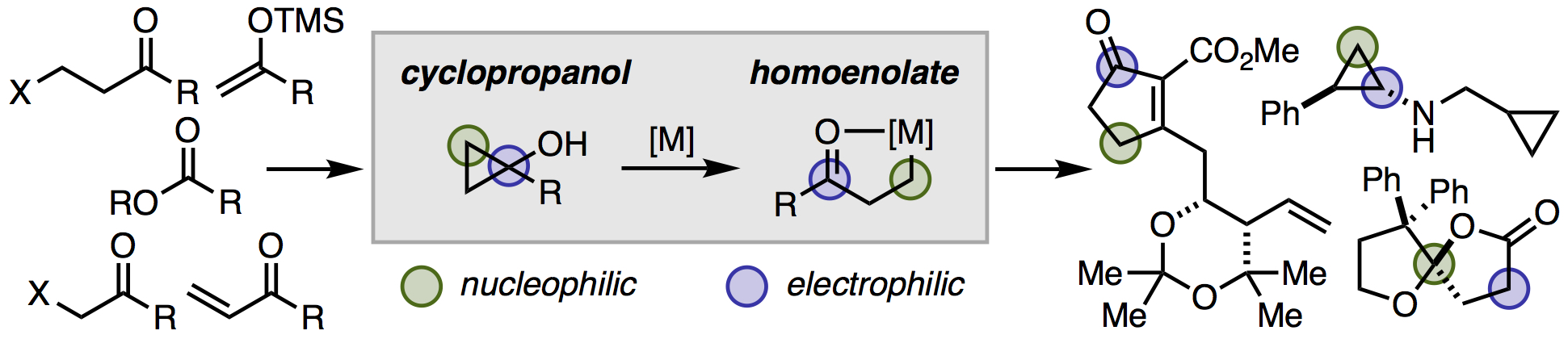
17. Mills, L. R.; Rousseaux, S. A. L. "Modern Developments in the Chemistry of Homoenolates" Eur. J. Org. Chem. 2019, 8-26. Cover Feature.
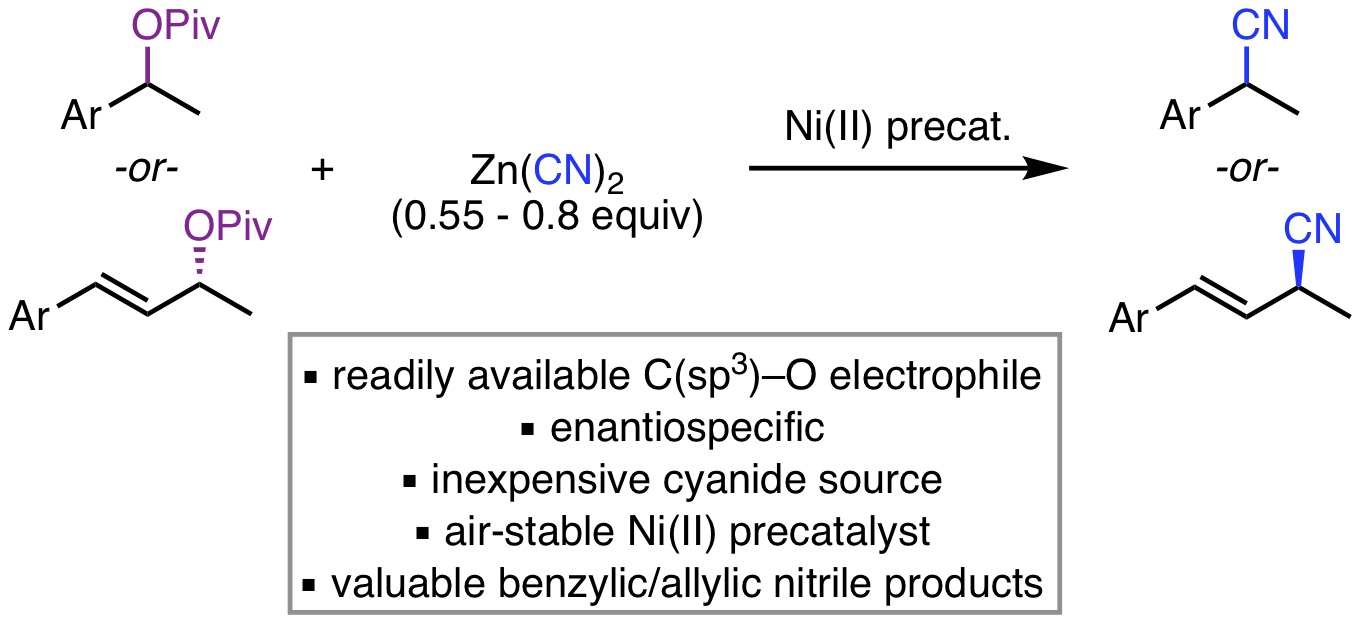
16. Michel, N. W. M.; Jeanneret, A. D. M.; Kim, H.; Rousseaux, S. A. L. "Nickel-Catalyzed Cyanation of Benzylic and Allylic Pivalate Esters" J. Org. Chem. 2018, 83, 11860-11872.
15. Mills, L. R.; Rousseaux, S. A. L. "Electrophilic Metal Homoenolates and Their Application in the Synthesis of Cyclopropylamines" Synlett 2018, 29, 683-688.
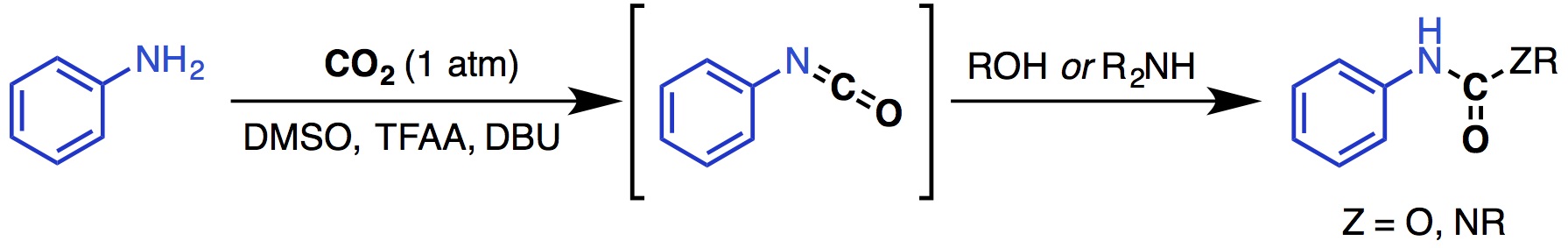
14. Ren, Y.; Rousseaux, S. A. L. "Metal-Free Synthesis of Unsymmetrical Ureas and Carbamates from CO2 and Amines via Isocyanate Intermediates" J. Org. Chem. 2018, 83, 913-920.
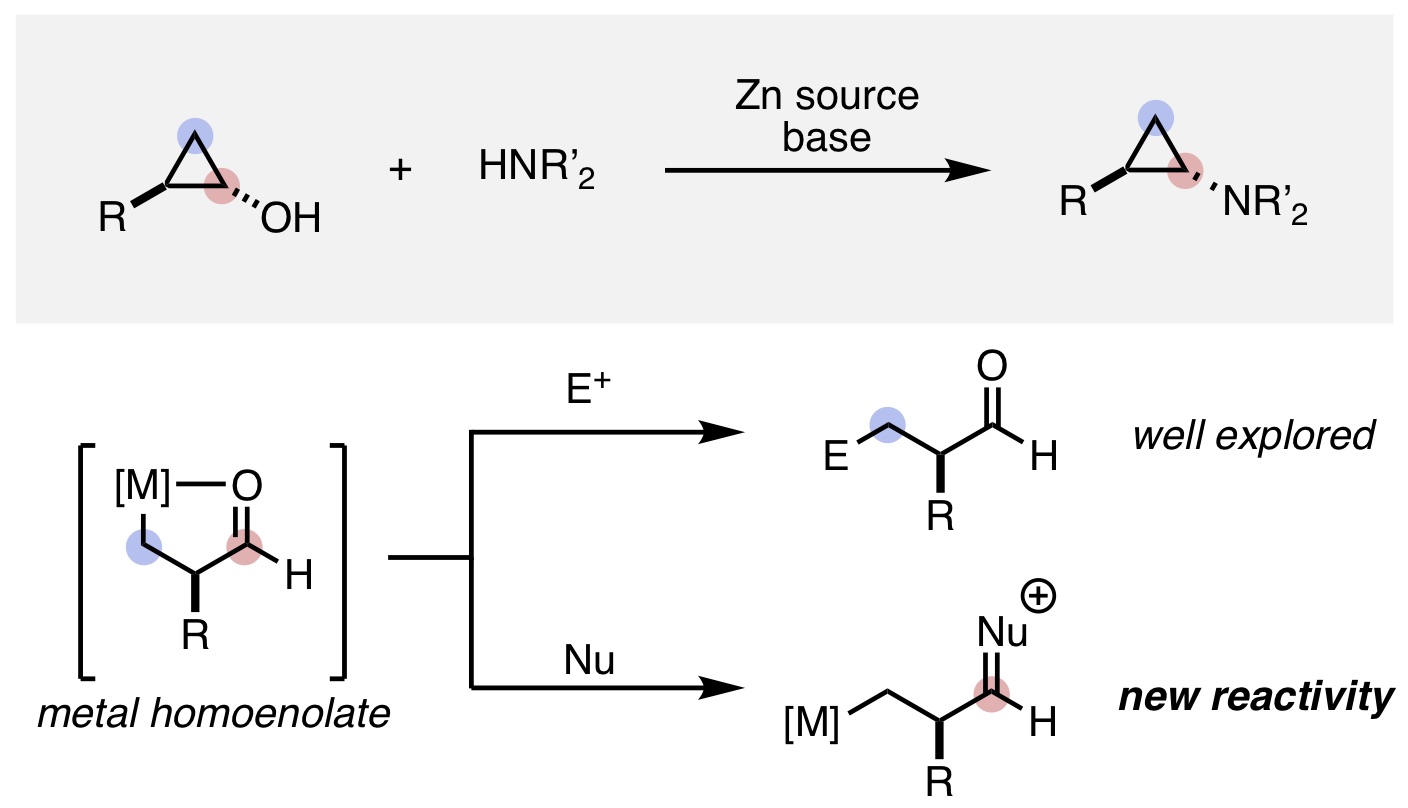
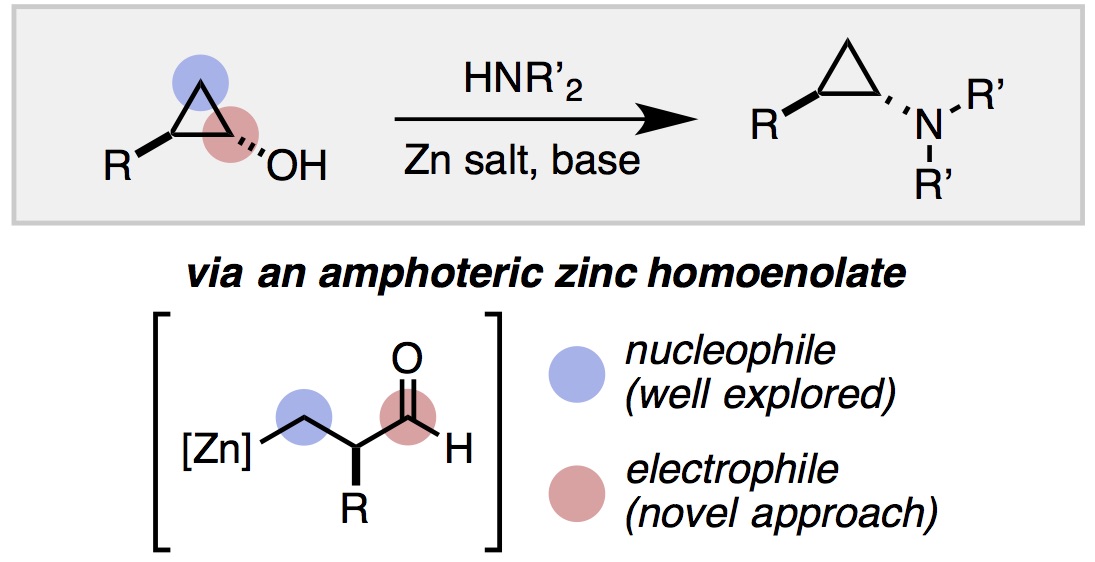
13. Mills, L. R.; Barrera Arbelaez, L. M.; Rousseaux, S. A. L. "Electrophilic Zinc Homoenolates: Synthesis of Cyclopropylamines from Cyclopropanols and Amines" J. Am. Chem. Soc. 2017, 139, 11357-11360.
Before UofT
12. Rousseaux, S. A. L.; Gong, J. Q.; Haver, R.; Odell, B.; Claridge, T. D. W.; Herz, L. M.; Anderson, H. L. "Self-Assembly of Russian Doll Concentric Porphyrin Nanorings" J. Am. Chem. Soc. 2015, 137, 12713-12718.
11. Liu, S.; Kondratuk, D. V.; Rousseaux, S. A. L.; Gil-Ramirez, G.; O'Sullivan, M. C.; Cremers, J.; Claridge, T. D. W.; Anderson, H. L. "Caterpillar Track Complexes in Template-Directed Synthesis and Correlated Molecular Motion" Angew. Chem. Int. Ed. 2015, 54, 5355-5359.
10. Candy, M.; Rousseaux, S. A. L.; San Román, A. C.; Szymczyk, M.; Kafarski, P.; Leclerc, E.; Vrancken, E.; Campagne, J.-M. "Palladium-Catalyzed Hydrophosphonylation of Alkenes with Dialkyl H-Phosphonates" Adv. Synth. Catal. 2014, 356, 2702-2708.
9. Rousseaux, S.; Vrancken, E.; Campagne, J.-M. "Chiral Aryl-Copper(III) Electrophiles: New Opportunities in Catalytic Enantioselective Arylations and Domino Processes" Angew. Chem. Int. Ed. 2012, 51, 10934-10935.
8. Rousseaux, S.; Liégault, B.; Fagnou, K. "C-H Functionalization: A New Strategy for the Synthesis of Biologically Active Natural Products" in Modern Tools for the Synthesis of Complex Bioactive Molecules (Eds. Cossy, J.; Arseniyadis, S.), Wiley-VCH, 2012, 1-32.
7. Rousseaux, S.; Liégault, B.; Fagnou, K. "Pd(0)-Catalyzed Cyclopropane C-H Bond Functionalization: Synthesis of Quinoline and Tetrahydroquinoline Derivatives" Chem. Sci. 2012, 3, 244-248.
6. Rousseaux, S.; García-Fortanet, J.; Del Aguila Sanchez, M. A.; Buchwald, S. L. "Palladium(0)-Catalyzed Arylative Dearomatization of Phenols" J. Am. Chem. Soc. 2011, 133, 9282-9285.
5. Rousseaux, S.; Davi, M.; Sofack-Kreutzer, J.; Pierre, C.; Jazzar, R.; Kefalidis, C.; Clot, E.; Fagnou, K.; Baudoin, O. "Intramolecular Palladium-Catalyzed Alkane C-H Arylation from Aryl Chlorides" J. Am. Chem. Soc. 2010, 132, 10706-10716.
4. Rousseaux, S.; Gorelsky, S. I.; Chung, B. K. W.; Fagnou, K. "Investigation of the Mechanism of C(sp3)-H Bond Cleavage in Pd(0)-Catalyzed Intramolecular Alkane Arylation Adjacent to Amides and Sulfonamides" J. Am. Chem. Soc. 2010, 132, 10692-10705.
3. Schipper, D. J.; Rousseaux, S.; Fagnou, K. "Kinetic Resolution of Quaternary and Tertiary β-Hydroxy Esters" Angew. Chem. Int. Ed. 2009, 48, 8343-8347.
2. Blaquiere, N.; Shore, D. G.; Rousseaux, S.; Fagnou, K. "Decarboxylative Ketone Aldol Reactions: Development and Mechanistic Evaluation Under Metal-Free Conditions" J. Org. Chem. 2009, 74, 6190-6198.
1. Campeau, L.-C.; Rousseaux, S.; Fagnou, K. "A Solution to the 2-Pyridine Cross-Coupling Problem: Regioselective Catalytic Direct Arylation of Pyridine N-Oxides" J. Am. Chem. Soc. 2005, 127, 18020-18021.