The Polymer and Colloids Group
 My group is interested in all aspects of this process, but our greatest contribution has been in developing new tools to study the final stage, in which polymer molecules diffuse across the interfaces between cells in the void-free solid film. This is the step that builds mechanical strength into the coating. Over the past 15 years, we have worked with paint companies and latex manufacturers around the world to help build a deeper understanding of the latex film formation process. [see Macromolecules, 37, 5752-5761 (2004).]
My group is interested in all aspects of this process, but our greatest contribution has been in developing new tools to study the final stage, in which polymer molecules diffuse across the interfaces between cells in the void-free solid film. This is the step that builds mechanical strength into the coating. Over the past 15 years, we have worked with paint companies and latex manufacturers around the world to help build a deeper understanding of the latex film formation process. [see Macromolecules, 37, 5752-5761 (2004).]
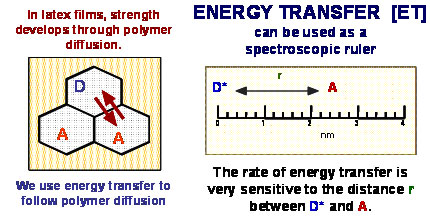
We are best known for our use of fluorescence resonance energy transfer (FRET or, simply, ET) measurements to study the rate at which polymer molecules diffuse across interfaces in latex films. We synthesize latex particles with nanometer dimensions with covalently bound dyes to act as donors or acceptors. Through our interactions with our industrial partners, we use this methodology to study how various factors (solvent additives, blends of hard and soft nanoparticles, the introduction of crosslinking chemistry into the polymer particles) affect this key step in the formation of useful properties. Our most recent project, in collaboration with scientists at Rohm and Haas, investigates the earliest stages of film formation as latex dispersions dry.
 Each year, the Federation of Societies for Coating Technologies holds its Roon Award competition for the best scientific contribution in the coatings area. We have won First Prize three times: in 1991 for our paper examining the effect of coalescing aids on polymer diffusion rates in latex films [see J. Coatings Technol., 64(811), 51-61 (1992)]; in 1995 for our paper examining the drying mechanism of latex blends [see J Coatings Technol., 68(852), 39-50 (1996)]; and 1998 for our paper on melamine derivatives as crosslinkers in an acrylic latex designed as a model for automotive basecoat [see J. Coatings Technol., 71(892), 47-60 (1999).].
Each year, the Federation of Societies for Coating Technologies holds its Roon Award competition for the best scientific contribution in the coatings area. We have won First Prize three times: in 1991 for our paper examining the effect of coalescing aids on polymer diffusion rates in latex films [see J. Coatings Technol., 64(811), 51-61 (1992)]; in 1995 for our paper examining the drying mechanism of latex blends [see J Coatings Technol., 68(852), 39-50 (1996)]; and 1998 for our paper on melamine derivatives as crosslinkers in an acrylic latex designed as a model for automotive basecoat [see J. Coatings Technol., 71(892), 47-60 (1999).].